Atoms & Heat
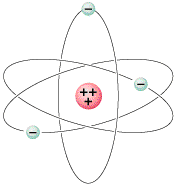 |
Chemical elements exist because different atoms can have differing numbers of protons and electrons. Hydrogen has one proton and electron; helium has two protons and two electrons; lithium has three protons and three electrons, etc. These are elements one, two, and three respectively, because the number of the element always indicates the number of protons and electrons it has. The collection of all known elements is called the periodic table. The table is arranged in such a way that elements in the same column of the table have similar chemical properties.
Sizewise, if the nucleus of say, an iron atom, were the size of a marble, then it would weigh several billion tons, and its electrons would be orbiting it in "shells" about a mile away. In other words, an atom is mostly empty space. The "size" of an atom is determined by the size of the shells of its electrons.
As for neutrons, they are electrically neutral, almost exactly the same size and mass as a proton, and together with the protons form the nucleus of the atom. Since protons have positive charges, they strongly tend to fly apart (because like charges repel) and in fact, it is not possible for a nucleus to contain only protons unless you count hydrogen, which only has one proton. Neutrons help to bind the nucleus together, because they contribute attractive nuclear binding forces but do not contribute repulsive electrical forces. (This topic will be covered in much more detail when we come to nuclear physics.)The neutrons do not have anything to do with the chemical properties of an element. Atoms with the same number of protons, but differing numbers of neutrons, are alike in every way except for their weight. For example, the compound "heavy water" is exactly like water except that it contains deuterium (an atom that has one proton and one neutron) rather than hydrogen. Because the deuterium has one proton, it acts exactly like hydrogen chemically. The total atomic weight of water is 18 (2 hydrogens at 1 each, and one oxygen atom at a weight of 16), so the atomic weight of heavy water is (2 X 2 + 18) = 20. That's it. The only measurable difference between heavy water and ordinary water is their relative weight ratio of 18 versus 20 for a given volume.
Atoms can share electrons to form molecules. A molecule represents the smallest possible unit of everything which is not an element. (In other words, if it isn't listed in the periodic table of elements, then it is made up of molecules.) Molecules in turn exert attractive forces on each other to form bulk materials: solids, liquids, and gases. The only difference between solids, liquids, and gases is how strongly their molecules attract each other. Solids have very strong intermolecular bonds, to the extent that they essentially lock each other into place like the mortar between so many bricks. Gases have practically no intermolecular attraction at all. The atoms of a gas bounce around freely in space like a pack of ping-pong balls in a clothes dryer. Liquids are somewhere in between, and act roughly as though the molecules are tied together by loose strings.
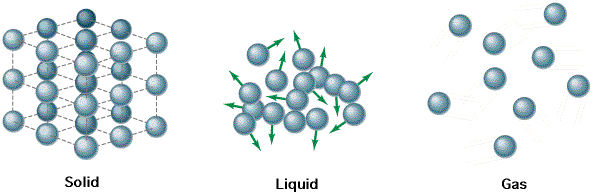
Heat is atomic motion. Nothing more. The atoms in a solid do not sit rigidly in one place, but rather, vibrate as though they are connected by springs. The more they vibrate, the more heat the solid is said to have. In the case of gases, more heat means that the "ping-pong balls" are moving at higher velocities. In both cases, as their atoms move faster, the temperatures of the materials will rise.
However -- temperature and heat are NOT the same thing. Heat is the total amount of kinetic motion in a substance, whereas temperature is the average amount of kinetic motion per atom. To see the difference, consider a red-hot nail and a bathtub full of tepid water. The nail has a much higher temperature than the water...but if I throw the nail into the bathtub, the nail instantly goes pfffst and cools down to room temperature, whereas the water is virtually unaffected. This is because the nail has the higher average kinetic energy (higher temperature), but the water contains far more atoms and thus far more total heat energy than the nail. When the nail and water meet, the transfer of heat energy cools the nail by hundreds of degrees, but that same transfer does almost nothing to the water's temperature, because the heat is being spread over too many molecules for any one of them to move much faster. Since temperature measures average motion, and the average motion of the water has barely been affected, the temperature of the water barely changes.
For an ideal gas (see the next page), kinetic energy is related to temperature (measured in K°) by:Not all materials absorb heat equally. If one places, say, equal amounts of water and alcohol into a microwave, and heats them with the same power and time settings, their final temperatures will not be the same. The alcohol will heat up more quickly (that is, its temperature will rise more swiftly) because it does not have the same capacity to absorb heat as does water. On an atomic level, this is because the strength of the "springs" connecting the atoms in alcohol and water are different, and thus the degree of vibration differs. The ability of a material to absorb heat is called its specific heat capacity. The total amount of heat in a material therefore depends on three things: the temperature, the mass of the material, and the ability of the material to absorb heat. These are all related by the equation:
1/2 mv2 = 3/2 kBT where kB is Boltzmann's Constant = 1.38 X 10-23 joule/K°
Q = mc (Tf - Ti), where:
Q is the amount of heat transferred to the material, m is the mass of the material, c is the material's specific heat capacity, Tf is the final temperature of the material (in degrees Centigrade or Kelvin), and Ti is the initial temperature of the material (in degrees Centigrade or Kelvin).
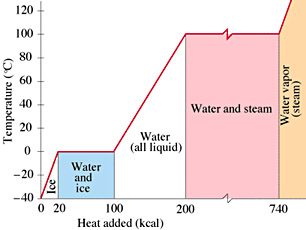 If one heats a solid enough, then it usually melts. This happens because the atoms begin vibrating so violently that the solid bonds between them break down and are converted into liquid bonds. Similarly, if a liquid is heated enough, then it eventually boils (turns into a gas) because the motion of the atoms becomes so great that they are no longer capable of holding on to each other. Curiously, if one monitors the temperature of a solid as it is heated, it will follow the equation for heat capacity until it starts to melt. Right at the melting point, the temperature suddenly remains constant, even though you are still adding heat. (See the diagram at right.) The reason for this is that it takes energy to convert the solid bonds to liquid ones, and all the heat is being directed into that, not into a rise in temperature. Only after all of the solid has been converted into liquid will the temperature begin to rise again. Similarly, a pot of boiling water does not change temperature as you add heat to it. All the heat is directed into breaking the liquid bonds, not into a temperature increase. If you add more heat, the water will boil more furiously....but it won't get any hotter. In short, the heat capacity equation is only good when the material is not undergoing a phase change: that is, not melting, freezing, boiling, or condensing.
If one heats a solid enough, then it usually melts. This happens because the atoms begin vibrating so violently that the solid bonds between them break down and are converted into liquid bonds. Similarly, if a liquid is heated enough, then it eventually boils (turns into a gas) because the motion of the atoms becomes so great that they are no longer capable of holding on to each other. Curiously, if one monitors the temperature of a solid as it is heated, it will follow the equation for heat capacity until it starts to melt. Right at the melting point, the temperature suddenly remains constant, even though you are still adding heat. (See the diagram at right.) The reason for this is that it takes energy to convert the solid bonds to liquid ones, and all the heat is being directed into that, not into a rise in temperature. Only after all of the solid has been converted into liquid will the temperature begin to rise again. Similarly, a pot of boiling water does not change temperature as you add heat to it. All the heat is directed into breaking the liquid bonds, not into a temperature increase. If you add more heat, the water will boil more furiously....but it won't get any hotter. In short, the heat capacity equation is only good when the material is not undergoing a phase change: that is, not melting, freezing, boiling, or condensing.
Here is a Quicktime movie that illustrates the temperature change of water as it is melted from ice and boiled into steam. (Keep your eye on the thermometer in the upper left-hand corner.)
And finally, we have to note that the interactions between atoms are very complex, and therefore there are plenty of exceptions to the general picture outlined above. For example, some solids (such as dry ice and moth balls) do not form liquids. If you heat them, they just convert directly to gases. As another example, many organic compounds are composed of molecules so fragile that when you heat them up, they just come apart. Bread left too long in a toaster does not melt into liquid bread. It essentially disintegrates into charcoal, because organic materials consist mostly of carbon, oxygen, and hydrogen. When these molecules disintegrate, the oxygen and hydrogen simply fly away as gases, and only the elemental carbon (otherwise known as charcoal) is left behind.
 Conservation Laws
|
Ideal Gas Law
Conservation Laws
|
Ideal Gas Law 