Dateline for Thermodynamics
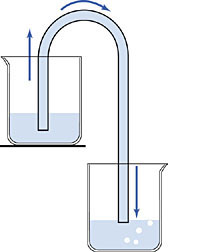
1643 A siphon is a fluid-filled tube that allows fluids at a higher level to flow to a lower level. As shown at left, a siphon will work even if the fluid first has to flow upwards before it eventually flows down to the bottom. A siphon is a neat way to move a liquid over a barrier with no work and no pumping. Siphons were widely used in Galileo's day to pump water out of mines, for example.
But, there is one major problem with siphoning water. A water siphon will only work if the barrier you're going over is less than 33 feet high. Beyond that, you get no flow. Aristotle had explained this limit by declaring that "nature abhors a vacuum", i.e., Aristotle did not think that a vacuum could exist. Sucking the air out of a tube would therefore naturally cause any liquid that might be there to be drawn towards the void, because nature must act to prevent a vacuum. The 33-foot limit represented the maximum force that the vacuum could exert on the water, in Aristotle's view.
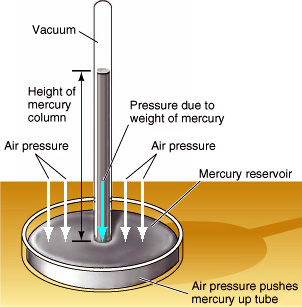 In 1643 Evangelista Torricelli, who had studied under Galileo, began wondering if the 33-foot limit had more to do with the weight of the air outside the siphon than with any supposed vacuum force inside the siphon. Could it be, Torricelli wondered, that a 33-foot-high column of water had exactly the same weight as a column of air extending to the top of the Earth's atmosphere? If so, then what a siphon really represented was a
In 1643 Evangelista Torricelli, who had studied under Galileo, began wondering if the 33-foot limit had more to do with the weight of the air outside the siphon than with any supposed vacuum force inside the siphon. Could it be, Torricelli wondered, that a 33-foot-high column of water had exactly the same weight as a column of air extending to the top of the Earth's atmosphere? If so, then what a siphon really represented was a Torricelli decided to test his theory by replacing the water with mercury, a heavy (and toxic) liquid metal that is 13.6 times as dense as water. If the weight of the fluid was all that mattered, then the mercury would rise 13.6 times less than the water, or about 33 / 13.6 = 2.5 feet.
Torricelli took a six-foot tube filled with mercury and turned it upside down into a bowl of mercury. (See illustration at right.) Sure enough, the mercury within the tube did NOT remain at a height of six feet, but fell instantly to a level about 2.5 feet above the bowl, in agreement with what Torricelli thought would happen. The resulting 3.5 feet of empty space above the mercury in the tube had to be a vacuum, because there was no way for anything else to get in. This proved that Aristotle was wrong in believing that a vacuum was impossible.
Torricelli later noticed that the height of the mercury varied slightly from day to day, which he correctly attributed to fluctuations in atmospheric pressure. He had invented the barometer. To this day, weathermen still report the daily atmospheric pressure in terms of inches, centimeters, or millimeters of mercury.
1645 Otto von Guericke, Mayor of Magdeburg, Germany, and a sometime physicist, hears about Torricelli's work and invents the first air pump. He uses it to evacuate a glass jar and demonstrate that a feather and a ball do indeed fall at the same acceleration in a vacuum, as Galileo had predicted.
1648 Blaise Pascal, a French mathematician and physicist whose health is not robust, talks his brother-in-law into carrying a barometer to the top of some nearby mountains to see if the mercury falls. It does. This is additional proof of Torricelli's thoughts about atmospheric pressure.
 1654 von Guericke stages a grand show for the Holy Roman Emperor Ferdinand III, as illustrated here, wherein two brass hemispheres about four feet in diameter are fitted together and a vacuum is created inside by means of von Guericke's pump. Two teams of horses are unable to pull the spheres apart, but the spheres literally fall apart when von Guericke opens a stopcock and lets the air back in. The Emperor is impressed.
1654 von Guericke stages a grand show for the Holy Roman Emperor Ferdinand III, as illustrated here, wherein two brass hemispheres about four feet in diameter are fitted together and a vacuum is created inside by means of von Guericke's pump. Two teams of horses are unable to pull the spheres apart, but the spheres literally fall apart when von Guericke opens a stopcock and lets the air back in. The Emperor is impressed.Since pressure = force per unit area (P=F/A), we can easily see why the horses failed. The total force on the spheres is F = PA, and in this case we have:
P = pressure of Earth's atmosphere = 14.7 lbs/in2
A = surface area of sphere = 4
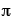 r2
r2= 4(3.14)(24 in)2 = 7,234 in2
F = (14.7 lbs/in2)(7234 in2)/(2000 lbs per ton) = 53 tons
(Actually, for reasons arising from the vector addition of forces around a sphere, the horses would only have had to supply half of 53 tons to pull the spheres apart, but never mind. The basic idea is correct.)
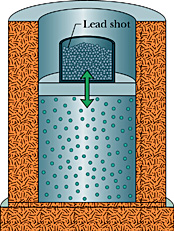 1662 Robert Boyle, a Scottish physicist, experiments by putting air in a sealed cylinder and then placing weights on it. He discovers that if he doubles the weight on the air, then it occupies half the volume; if he triples the weight, it occupies one-third the volume; etc. In other words:
1662 Robert Boyle, a Scottish physicist, experiments by putting air in a sealed cylinder and then placing weights on it. He discovers that if he doubles the weight on the air, then it occupies half the volume; if he triples the weight, it occupies one-third the volume; etc. In other words:PV = constant, where P is the pressure of the gas, and V is the volume occupied by the gas. This becomes known as Boyle's Law. Boyle speculates that air of consists of atoms with empty space between them, and that compression is possible because the atoms are being pushed closer together.
1679 Denis Papin, French physicist and amateur gourmet, invents the pressure cooker. The high pressure inside the cooker creates super-heated
1687 Isaac Newton publishes the Principia.
1698 Thomas Savery, an English merchant, invents the "Miner's Friend". This is basically a boiler attached to a long tube, whose purpose is to pump water out of mines. The idea is to create high-pressure steam in the boiler and force it down the pipe, whereupon it can drive the water up another pipe to the surface. It is a commercial failure. The metal-working of the time cannot handle the high pressure; the steam condenses in the uninsulated tubes before it can do any useful work; etc. But, it starts some people to thinking about steam, and in particular, it starts Thomas Newcomen to thinking about steam.
1709 Abraham Darby, an English iron manufacturer, discovers how to use coal instead of charcoal for smelting iron. (I am referring to the process where carbon is mixed into the molten iron to help refine it; I am not discussing how the iron is heated and melted. Both charcoal and coal are mostly
1712 The mining industry in England has reached a near-crisis situation; underground flooding is limiting the amount of mining which can take place, even though the demand for iron, coal, and tin is sharply rising. Thomas Newcomen, an English iron merchant who is well aware of the problem, builds his first Fire Engine, which he has spent nearly twelve years perfecting.
His engine consists of one massive cylinder, about 4 feet in diameter and 15 feet high, which sets atop a water boiler. A brick wall is used to support a massive rocking
The force of the downstroke can be estimated from the formula F = PA. The area of the top of the cylinder is A =This is a lot of force. Finally, there is a way to lift large quantities of water from deep mines. The Newcomen engine operates at a surprising 12 strokes per minute...but certainly not in a smooth fashion! The rocking arm basically free-falls in both directions, with a lot of booming and banging, and is distressingly prone to tearing itself apart if the chains shouldr2, so we have:
F = (14.7 lbs/in2)(3.14)(24 in)2 = 26,587 lbs, or roughly 13 tons.
The manufacturing technology of the time is just barely able to make such large cylinders with the necessary precision to be air-tight, and the Newcomen machines cost the modern equivalent of several million dollars each. However, they do work. Several dozen are built over the next decade, and over 400 are in use by the time of the American Revolution. By 1800 there are nearly 1,500 Newcomen engines swinging above English iron, coal, and tin mines, and their presence has become as much a part of the English landscape as oil wells are today in Saudi Arabia.
1738 The Swiss mathematician and physicist Daniel Bernoulli formulates his kinetic theory of gasses. He assumes that gases consist of hard particles in a vacuum which are continuously moving and bouncing off each other and their surroundings like perfectly elastic kernels in a popcorn popper. In this view, the air pressure on something is created by the innumerable "pinging" of enormous numbers of gas atoms bouncing against it. Bernoulli is able to show mathematically that such a cloud of atoms would obey Boyle's Law, PV = constant.
However, his theory is almost ignored. Atoms are still considered too speculative by many of Bernoulli's contemporaries, and Bernoulli has no way to prove their existence. Furthermore, many physicists believe that atoms (if they exist at all) would have to operate like the planets, and move slowly through space while interacting with each other by long-range forces. Isaac Newton's Principia has been so successful in explaining large-scale motion that nearly everyone believes that it must apply to small-scale motion as well. Little ping-pong balls whizzing around with no interaction forces at all must wait for more evidence in their favor.
1760 Joseph Black, a Scottish chemist and physicist who has friends in the beer-brewing business, has been asked by them to investigate why it seems to take so much coal to boil water. Can he figure out a way to save them money? Black does some investigating, and discovers the basic law of heat capacity, given by the formula:
Q = mcs
T, where
Q is the amount of heat transferred to or from the substance, measured in calories
m is the mass of the substance, measured in grams
cs is the specific heat capacity of the substance, measured in calories per gram °C,
T is the change in temperature of the substance, measured in °C
Black considers his discovery to be evidence for the existence of caloric,the theoretical fluid which is thought to mediate heat exchange. According to the theory, heat exchange is caused by caloric flowing from hot bodies to cold ones. Black hypothesizes that different materials have differing heat capacities because their ability to "hold" caloric is different.
1762 Joseph Black discovers the concept of latent heat, which is the additional energy (over and above the heat capacity) which must be supplied to solids in order to melt them, or to liquids in order to boil them. (Similarly, this same amount of latent heat must be taken out in order to condense a gas into a liquid, or to freeze a liquid into a solid.) Black regretfully tells his friends in the beer business that there is nothing anyone can do to make the latent heat of water go away so that they can use less coal to boil their water.
1764 James Watt, a Scottish engineer working at the University of Edinburgh, is asked to repair a model Newcomen engine which the University owns. He notices that it is incredibly fuel-inefficient. (NOTE - engines of the Newcomen type are about 1% efficient, that is, out of every 100 joules of heat energy released by the burning coal, only one joule goes into lifting water, and the other 99 go into warming the great outdoors.)
Watt discusses the matter with Black, who is a professor at the University, and learns of heat capacity. Watt realizes that the Newcomen design, which alternately fills the same cylinder with hot steam and cold water, is wasting a dreadful amount of energy on continuously reheating and recooling the iron. He begins work on a design in which the steam will be released from the power cylinder after use and condensed in a second chamber.
1783 The Montgolfier brothers (aka the Wright brothers of the Age of Reason) launch the first hot-air balloon. It is said that an onlooker asked one of the brothers, "Of what use is this?", and came the reply, "Of what use is a newborn baby?"
In August of that same year Jacques Charles, a French physicist, realizes that hydrogen gas will provide much more lift and also provide permanent buoyancy, and so launches the first hydrogen balloon.
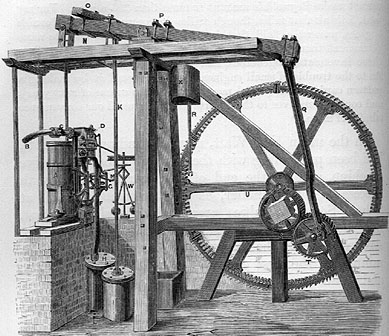 1784 For the last ten years Watt has been in financial partnership with Matthew Boulton, a Birmingham merchant and entrepreneur. Boulton believes that an economical steam engine capable of providing constant, rotative power (similar to a water wheel) would have wide applications in the textile industry. Watt solves the last of the practical problems associated with such a machine and in 1784, although no one knows it yet, the Industrial Revolution has begun. Watt's design features:
1784 For the last ten years Watt has been in financial partnership with Matthew Boulton, a Birmingham merchant and entrepreneur. Boulton believes that an economical steam engine capable of providing constant, rotative power (similar to a water wheel) would have wide applications in the textile industry. Watt solves the last of the practical problems associated with such a machine and in 1784, although no one knows it yet, the Industrial Revolution has begun. Watt's design features:
1) A separate cold-water chamber for condensing the steam from the main cylinder. This innovation makes Watt's engine 4 to 5 times more fuel-efficient than the older Newcomen engines. Boulton & Watt market their machine by charging a royalty on the fuel savings achieved by the customer as compared to using a Newcomen engine.
2) Double-action piston. Watt has invented an ingenious arrangement of valves which admits steam first on one side of the piston, then on the other. This double-action gives the Watt engine more power than the Newcomen design and also makes the power output smoother.
3) Sun and planet gearing. By using a heavy wheel with a "sun and planet" gear, Watt is able to convert the up-and-down action of the piston into the rotary motion necessary for running looms and spinning wheels. The large angular momentum of the wheel also serves as an aid in smoothing out the power delivery of the engine.
4) Centrifugal governor. The centrifugal force from a pair of rotating balls is used to control a rod connected to the steam valves, to help regulate the speed at which the steam engine runs.
The Watt design, however, like the Newcomen design, works at atmospheric pressure. The steam serves only a passive role, allowing the piston to be moved prior to creation of a vacuum by condensation of the steam. The power is generated entirely by air pressure acting on the piston.
1787 Jacques Charles discovers that the volume of a gas whose temperature is near the freezing point of ice (0 °C) changes by the fraction 1/273 for each degree centigrade of temperature change. That is:
, where:
V = new volume of the gas, V0 = original volume of the gas,T = change in temperature in °C
It is immediately realized, of course, that if this formula were to hold for all temperatures, then the gas would disappear altogether at -273 °C !!! This does not seem plausible, but without artificial refrigeration there is no way of directly testing the idea in 1787.
1789 Antoine Lavoisier, an aristocratic French chemist who is generally considered to be the most important chemist in history for his many fundamental discoveries, enunciates the principle of conversation of mass.
1790 The English government, quick to realize the enormous economic advantages which the technology of the Industrial Revolution is giving England, has outlawed the export of any books, drawings, or descriptions of the new machinery. Also, experts in the new technology are forbidden to leave the country (shades of the Iron Curtain). Samuel Slater, a young engineer, is aware that he can advance only so far in the class-ridden social structure of England, and that rich merchants in America are willing to pay handsomely for his knowledge. He painstakingly memorizes every detail of the machinery in a textile mill, and escapes to America disguised as a farm laborer. The Industrial Revolution thus comes to Pawtucket, Rhode Island.
1794 Antoine Lavoisier is executed by the French Revolution. When an international appeal is made on his behalf, the petitioners are given the reply: "The State needs no scientists."
1798 Count Rumford, the Director of the Bavarian arsenal, is responsible for the boring of cannon. He notices that the water used to cool the cannons as they are being bored is carrying off so much heat that it is nearly boiling. Where could all this heat be coming from? The caloric theory says that it is coming from the iron shavings, which are considered to have less capacity for holding caloric than larger pieces of iron, but Rumford is skeptical. He has noticed that the water continues boiling and boiling even after the drill bits have become so dull that they aren't producing any shavings at all. He proposes that the energy of motion of the drill itself is being converted into heat, and that the caloric fluid does not exist.
Rumford, in a truly eclectic career which includes work as a schoolmaster, explorer, secret agent, and army officer, also invents the drip coffeepot, the built-in kitchen, the pencil eraser, and thermal underwear, the latter of which earns him a medal from the Royal Society.
1801 John Dalton, an English chemist, has noticed that chemical elements always form compounds with certain weight ratios. Dalton proposes that this is because the elements consist of atoms which have certain weights, and therefore are forming compounds which must reflect those weights.
1802 Joseph Gay-Lussac, a French physicist, independently discovers Charles' Law (see 1787) and realizes that it can be combined with Boyle's Law to produce a single formula for gases:
PV = (const) (T + 273), where
P = pressure
V = volume
T = temperature in °C
This is called the Ideal Gas Law, or sometimes the Perfect Gas Law. (It turns out that this law is in fact an approximation, and no real gas follows the Ideal Gas Law exactly....but in most situations, it is more than good enough.)
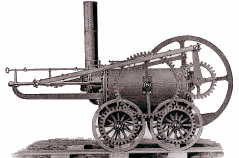 1804 For the past 20 years, following the introduction of the Watt steam engine, scientists and engineers have been working to make steam engines lighter, more rugged, and most importantly,
1804 For the past 20 years, following the introduction of the Watt steam engine, scientists and engineers have been working to make steam engines lighter, more rugged, and most importantly, 1807 The American inventor Robert Fulton installs a steam engine on the boat Clermont, and begins the world's first financially successful steamboat service.
1811 Amadeo Avogadro, an Italian chemist, is puzzled by the discrepancies between conflicting theories of how gases chemically combine within the Dalton atomic theory. He eventually hypothesizes that all samples of gases whose volumes, temperatures, and pressures are equal contain exactly the same number of molecules. As time goes by, chemists and physicists gradually realize that Avogadro must be right. The constant in the ideal gas law is identified as being equal to NkB, where N is the number of molecules in the gas sample, and kB must be a fundamental constant equal for all gases. This constant is now called Boltzmann's Constant, in honor of Ludwig Boltzmann, for his fundamental theoretical work on statistical mechanics.
1824 Inventors working on developing small steam engines for locomotives and boats had, of course, begun to use high-pressure steam in their engines, as opposed to the atmospheric-pressure steam of Watt's engines. (They needed more power, and pushing a piston with 100 lbs/in2 of steam pressure rather than 14.7 lbs/in2 was a good way to get it.) As a side effect, they had noticed an astonishing thing: their high-pressure engines were far more fuel efficient than a Watt engine, upwards of three times as efficient. It is a complete mystery as to why.
In 1824 the French physicist and engineer Sadi Carnot publishes "On the Motive Power of Fire", in which he lays out the basic principles by which heat is converted into mechanical work. He shows that the maximum possible fuel efficiency for any steam engine depends only on the temperatures over which it operates. That is, if you could construct an ideal engine, one free of friction and perfectly heat-insulated and whose construction was such that it could extract every possible iota of usable energy from the steam, then its efficiency would be given SOLELY by the temperature of the steam as it goes into the piston and the temperature of the steam as it comes out. That's all. Using modern notation, we can say that the maximum possible efficiency e of any heat engine operating between two temperatures is:
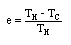 , where TH = the higher temperature, and TC = the colder temperature, in °K.
, where TH = the higher temperature, and TC = the colder temperature, in °K.This result explained why the high-pressure engines were so much more fuel-efficient than the Watt engines. Highly pressurized steam also means higher-temperature steam (see the entry for 1679), which gives a higher possible theoretical efficiency. Given two steam engines in which friction and so forth are more-or-less equivalent, then the higher-pressure engine will have the better efficiency.
Once this was realized, engineers began aggressively pursuing designs for higher and yet higher pressure steam engines, as they realized that this would lead to both greater power and fuel efficiency
1843 James Joule, a Scottish physicist, carefully measures the temperature increase in extremely well-insulated bottles of water which are being stirred by paddles driven by falling weights. (Click for illustration.) He determines that one calorie of heat is equal to 4.186 joules of gravitational potential energy. (Well, not exactly. The joule hadn't been created and named after Joule, yet. Joule did his work in English units, of course, and a later generation translated it to metric.)
1847 The principle of the universal conservation of energy is first enunciated by Hermann Helmholtz, a German physicist. The concept had been crystallizing for the past 50 years. Any number of people had proposed energy-conservation principles for heat transfer, elastic collisions, etc., but Helmholz gets credit for putting the idea onto a firm scientific footing and for proposing that ALL energy...chemical, radiant, gravitational, heat, kinetic, latent, you name it...can be converted into other forms with the total remaining constant.
1848 William Thompson, a British physicist also known as Lord Kelvin, realizes that Charles' Law does not really predict that the volume of an ideal gas will go to zero at -273 °C, but rather, that the thermal motion of the atoms will come to a stop at -273 °C. This is a much more meaningful idea. Kelvin proposes that this be called absolute zero, and introduces a new temperature scale, now known as the Kelvin scale, which is equal to the centigrade scale except that the zero point is moved down 273 degrees. Stated in terms of absolute temperatures, the Ideal Gas Law becomes:
PV = NkBT, where
P = the pressure of the gas sample
V = the volume of the gas sample
N = the total number of molecules in the gas sample
T = the absolute temperature, measured in °K
kB = Boltzmann's constant, which is 1.38066 X 10-23 joule/°K
1850 Rudolf Clausius, a German physicist, formulates what is now known as the 2nd Law of Thermodynamics. This is essentially a more general and more mathematically precise formulation of the principles deduced by Carnot for the maximum possible efficiency of a heat engine. In general, the 2nd Law states that the Universe is statistically more likely to move towards disorder. By "move to disorder", we mean that the Universe tends to randomize any system that it can. If you use some red food coloring to "mark" the top half of a glass of water, that ordered state between red water and clear will not last long. The system will move towards disorder, i.e., towards a state where the entire glass is one shade of pink.
In detail, the 2nd Law states that it's not an absolute requirement that heat flow from hot to cold, but rather, that this is the
most probable outcome if hot and cold are placed together. Intuitively, the concept is simple. Suppose I have a frictionless pool table with some slow-moving billiard balls rolling on it. If I fire a fast-moving billiard ball onto the table, what will probably happen? Will the fast-moving ball somehow collide with the others in such a way as to increase its velocity, and rocket off the table even faster than it came on? Or will it bang around and muddle around with the slow-moving balls until it finally mixes in and ceases to be fast-moving? Your intuition is correct: the "hot" billiard ball will slow down and become a "cold" billiard ball. In a nutshell, this is why "hot" always flows towards "cold" in any thermodynamic system.
The 2nd Law of Thermodynamics is inherently a statistical law, but because it is normally applied to systems containing HUGE numbers of particles (a kilogram of anything contains roughly 100,000,000,000,000,000,000,000,000 atoms), the probability that a system won't obey the 2nd Law of Thermodynamics (i.e., the probability that heat will suddenly start running from cold to hot) is so vastly infinitesimal that the 2nd Law can be considered as absolute as any law in physics. The 2nd Law can be worded in a number of alternative ways. One popular way is to say that it is impossible to convert thermal energy into any other form of energy with 100% efficiency. (Please note that the reverse is not true. You can convert other forms of energy into heat with 100% efficiency.)
1859 Scottish physicist James Clerk Maxwell works out the kinetic theory of gases. Maxwell is able to put the older ideas of Bernoulli onto a much more rigorous theoretical footing, and derives a number of other important results. He demonstrates that the velocities of the molecules in an ideal gas must be distributed along a certain type of bell curve (now known as the Maxwell-Boltzmann Distribution), with the average velocity given by the formula:
1/2 mv2 = 3/2 kBTThe formula is written in this format to emphasize the relationship between the average kinetic energy of the molecules and the absolute temperature.
where:
m = the mass of the gas molecules (we assume there is only one type of molecule in the gas)
v = the average velocity of the molecules
T = the temperature of the gas, in °K
kB = Boltzmann's constant
 Refrigeration
|
Waves
Refrigeration
|
Waves 
A Ukrainian translation of this page is available here: Ukrainian