1853 - It has long been thought that the Earth is no more than a few tens of thousands of years old. Beginning in the 1820's, however, many geologists and biologists have come to believe that the Earth is much older than previously thought, perhaps in the hundreds of millions of years. (Darwin estimates the age of the Earth at 300 million years in the initial printing of Origin of Species.) These estimates are based on an increased awareness of how very slowly geological and biological processes such as erosion or evolution occur, and therefore how enormously old the Earth must be to accomodate them.
Prominent physicist William Thompson (also known as Lord Kelvin - degrees Kelvin are named after him) is firmly opposed to evolution. He begins to marshall theoretical evidence against Darwin. He performs classical thermodynamic calculations which prove that if the Earth were as old as Darwin and others claim, then it would have long since cooled to an inert rock and no geological activities such as volcanism or hot-water springs would be possible. Other physicists soon join the fray. Hermann Helmholtz, who only six years earlier had enunciated the principle of conversation of energy, calculates how much heat the Sun would radiate if its energy comes from slow contraction, thus converting gravitational potential energy into heat. He calculates an age of only 18 million years.
The enormous gap between geology and biology on the one hand, and theoretical physics on the other (as far as estimating the age of the Earth is concerned) will last for 50 years. In the face of the hard criticism from well-respected physicists, Darwin removes all mention of any specific age for the Earth in later printings of Origin of Species.
1896 - Henri Becquerel, a French physicist, reads of William Roentgen's experiments with X-rays, and learns that they can cause certain materials to fluoresce. (Tech Note - The X-rays were only exciting spectral lines in the fluorescent materials, like the gas tubes I show in class except with X-rays instead of electricity.)
Becquerel wonders, do phosphorescent materials emit X-rays while they are glowing? (Tech Note - They don't.) To test his idea, Becquerel obtains some materials which glow after being exposed to light, just like those magic decoder rings which they still put into cereal boxes. He conducts some experiments in which he first sets the materials out in the sun to start them glowing, then sets them on top of a photographic plate wrapped in black paper to see if they are emitting X-rays. Becquerel obtains some positive results, and some negative ones, which is confusing.
One day, when it's cloudy, he puts one of the minerals that has been giving him positive results into a drawer with an unexposed photographic plate - and then on a whim decides to develop it, expecting to see only a faint outline since the Sun was so dim that day. Instead, he accidentally discovers that the plate has become completely fogged even though the mineral had been barely exposed to light at all and wasn't glowing! The mineral happens to be potassium uranyl disulfate, and Becquerel eventually discovers that the uranium in this compound is the magic ingredient. All compounds with uranium in them will fog a photographic plate; compounds without uranium will not. Becquerel therefore calls the new radiation "uranic rays".
Tech Note - The property which makes some compounds "glow in the dark" after being exposed to light has to do with their molecular structure, and has nothing at all to do with either X-rays or with radioactivity. In brief, some molecules exhibit a marked "time delay" between when they are excited by incoming light, and when they emit their molecular spectral lines. Instead of instantly releasing all their stored energy and going out after the power is removed, like a neon sign does, phosphorescent materials gently release their energy for some while after the stimulus has been removed. It was sheer accident that Becquerel was using a "glow in the dark" compound which happened to have uranium in it.
1897 - Ernest Rutherford, a physicist originally from New Zealand but working in Canada, investigates Becquerel's "uranic rays" and discovers that they are in fact a mixture of two components: a very heavy component which is easily absorbed by matter and has a positive charge; and a much lighter, more penetrating component which is not so easily absorbed and has a negative charge. Rutherford calls these components 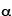 and
and 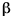 , after the first two letters of the Greek alphabet.
, after the first two letters of the Greek alphabet.
1898 - Pierre and Marie Curie, two French physicists who are studying Becquerel's "uranic rays", discover that thorium also gives off "uranic rays". They propose the new term "radioactivity" to describe elements which have the property of giving off rays. Working from samples of pitchblend, they isolate and discover two new elements which are much more intensely radioactive than uranium: the Curies name them polonium (after Marie's homeland of Poland) and radium (due to its highly radioactive power).
1899 - French chemist Andre Debierne, a close friend of the Curies, isolates yet another radioactive element from pitchblende. He calls it actinium, after the Greek word for ray.
Becquerel, who has continued to study "uranic rays", realizes that the b-particles of Rutherford are so much like electrons that they must be electrons, albeit electrons of very high energy.
The French physicist Paul Villard discovers that uranium is giving off yet a third component, one which is not affected by magnets and so is apparently uncharged. They are considerably more penetrating than either 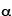 -particles or
-particles or
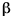 -particles, and Villard calls them (predictably)
-particles, and Villard calls them (predictably) 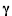 -rays, after the third letter of the Greek alphabet. Villard suspects that
-rays, after the third letter of the Greek alphabet. Villard suspects that 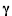 -rays are electromagnetic radiation of incredibly short wavelength, even shorter than X-rays. (He is right.)
-rays are electromagnetic radiation of incredibly short wavelength, even shorter than X-rays. (He is right.)
Tech Note - We still use the terms "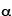 -particles", "
-particles", "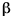 -particles", and
"
-particles", and
"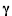 -rays" to refer to the three forms of radiation, even though we know that
-rays" to refer to the three forms of radiation, even though we know that 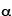 - and
- and 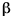 -particles are really just helium nuclei (two protons and two neutrons) and electrons, respectively.
-particles are really just helium nuclei (two protons and two neutrons) and electrons, respectively.
1901 - The Curies measure the energy being given off by radioactive elements, and discover that one gram of radium gives off the incredible amount of 140 calories per hour. As far as they can tell, this energy just magically goes on and on, undiminished, for month after month. The radium does not seem to be changing in any way. Where is all this energy coming from? Is conservation of energy being violated?
1903 - Ernest Rutherford is the first to realize that the long-standing dispute about the age of the Earth between biologists and geologists on the one hand, and physicists on the other, can be resolved if one assumes that the interior of the Earth contains slight traces of radioactive elements. The vast bulk of the Earth, and the poor thermal conductivity of the rocky materials that mostly constitute it, mean that even a small input of heat would be enough to keep it geologically active for far longer than the times calculated by William Thompson (who of course assumed that the Earth's interior was completely inert). Rutherford hypothesizes that the (apparently inexhaustable) energy produced by radioactive ores is in fact exactly that heat source, thus siding with the biologists and geologists concerning the age of the Earth.
Indeed, within only a few years, Rutherford and other physicists investigating radioactives ores come to the conclusion (based on the very long halflives of some of the isotopes they've found) that the age of the Earth may well be in the billions of years rather than the mere hundreds of millions. (They are right -- the currently accepted value for the age of the Earth is about 4.2 billion years.)
1906 - Rutherford discovers that 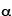 -particles, when brought to a stop inside a container, become helium atoms. In other words, an
-particles, when brought to a stop inside a container, become helium atoms. In other words, an 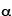 -particle consists of two protons and two neutrons (which is the nucleus of a helium atom) moving at high speed. If and when the
-particle consists of two protons and two neutrons (which is the nucleus of a helium atom) moving at high speed. If and when the 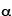 -particle is slowed down and captures a couple of electrons from somewhere, it becomes recognizable as ordinary helium.
-particle is slowed down and captures a couple of electrons from somewhere, it becomes recognizable as ordinary helium.
The very high speed of the helium nuclei, and the high speed of the electrons (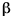 rays) emitted by radioactive elements, and the high-energy electromagnetic radiation also emitted, and the heat measurements by the Curies, indicate that there is something going on in these elements which is very energetic indeed. But what? Rutherford does not realize that the answer has already been published by Einstein in 1905 (indirectly), in the form of E = mc2.
rays) emitted by radioactive elements, and the high-energy electromagnetic radiation also emitted, and the heat measurements by the Curies, indicate that there is something going on in these elements which is very energetic indeed. But what? Rutherford does not realize that the answer has already been published by Einstein in 1905 (indirectly), in the form of E = mc2.
1909 - Eugene Marsden and Hans Geiger are two graduate students working with Ernest Rutherford in Manchester, England, where Rutherford has relocated. They perform a series of experiments in which 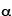 -particles are shot into a gold foil. Contrary to expectations, most of the
-particles are shot into a gold foil. Contrary to expectations, most of the 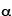 -particles go through the gold as if it wasn't there, but a few are deflected through large angles, and a very few even turn around and bounce straight back as though they have hit an impenetrable barrier. This leads Rutherford to propose the "solar system" model of the atom, in which the atom is essentially empty space but has a very small and incredibly dense nucleus. (See the Quantum Mechanics Timeline for more details.)
-particles go through the gold as if it wasn't there, but a few are deflected through large angles, and a very few even turn around and bounce straight back as though they have hit an impenetrable barrier. This leads Rutherford to propose the "solar system" model of the atom, in which the atom is essentially empty space but has a very small and incredibly dense nucleus. (See the Quantum Mechanics Timeline for more details.)
1913 - The British chemist Frederick Soddy and the American chemist Theodore Richards elucidate the concept of atomic weight. As people continued to study radioactivity, it had become increasingly clear that there were multiple varieties of elements. For example, there are both radioactive and nonradioactive versions of carbon. Soddy and Richards prove that the difference lies in the weight of the atomic nucleus - there can be different versions of the same element with different weights. The different versions are christened isotopes, from the Greek words meaning "same place".
Tech Note - The chemical properties of an element are determined solely by the number of protons in a nucleus, because it is the positively-charged protons which interact with the electron cloud around the nucleus, and it is the electron cloud which produces chemistry. Nuclei can also contain neutrons, which have about the same mass as protons but have no charge. Neutrons can thus affect the weight of a nucleus, and its radioactive properties, but have no effect on its chemical properties.
1915 - American chemist William Harkins notes that the mass of a helium atom is, in fact, not exactly four times that of a proton. It is slightly less. He states that the excess mass has been converted to energy via Einstein's E = mc2 and that this is the source of nuclear energy.
1919 - Rutherford, still hard at work bombarding things with 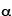 -particles (see 1897, 1906, 1909) succeeds in getting an
-particles (see 1897, 1906, 1909) succeeds in getting an 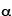 -particle (i.e., a helium nucleus) to react with a nitrogen nucleus to produce a proton (i.e., a hydrogen nucleus) and an oxygen nucleus. Rutherford has brought about the first human-engineered nuclear reaction. Also, this makes him the first person in history to change one element into another.
-particle (i.e., a helium nucleus) to react with a nitrogen nucleus to produce a proton (i.e., a hydrogen nucleus) and an oxygen nucleus. Rutherford has brought about the first human-engineered nuclear reaction. Also, this makes him the first person in history to change one element into another.
1930 - British physicist Paul Dirac is trying to combine relativity and quantum mechanics. He succeeds, and the relativistic quantum equation is called the Dirac equation as a consequence. He notices that his equation predicts the existence of "negative" states for the electron and proton, and he thus predicts the existence of antimatter.
1931 - For over a decade, physicists have been wrestling with a very puzzling problem with 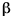 -emission. The electrons emitted by
-emission. The electrons emitted by 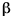 -decay do not always have the same kinetic energy, unlike the particles emitted in
-decay do not always have the same kinetic energy, unlike the particles emitted in 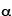 -decay. Rather, the electrons come off with a bell-curve-type distribution of energies, which means that (1) energy is apparently not being conserved, and (2) the amount of missing energy varies in some probablistic way. It seems that some of the nuclear energy powering
-decay. Rather, the electrons come off with a bell-curve-type distribution of energies, which means that (1) energy is apparently not being conserved, and (2) the amount of missing energy varies in some probablistic way. It seems that some of the nuclear energy powering 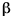 -decay is going someplace other than the emitted electron. But where? Elaborate attempts are made to detect heat or electromagnetic radiation coming from the samples -- but every effort fails. A few physicists begin to seriously wonder if perhaps
-decay is going someplace other than the emitted electron. But where? Elaborate attempts are made to detect heat or electromagnetic radiation coming from the samples -- but every effort fails. A few physicists begin to seriously wonder if perhaps 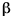 -decay really does violate conservation of energy, and Niels Bohr goes so far as to work out a possible scenario for how the Sun's energy could be generated by massive energy non-conservation resulting from
-decay really does violate conservation of energy, and Niels Bohr goes so far as to work out a possible scenario for how the Sun's energy could be generated by massive energy non-conservation resulting from 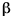 -decays.
-decays.
German physicist Wolfgang Pauli and Italian physicist Enrico Fermi propose that b-decay is producing two particles which share the kinetic energy: an electron, and an unseen particle which Fermi christens as a neutrino, from the Italian for "little neutral one". The particle is assumed to be very light as well as neutral, allowing it to penetrate matter so easily that it is almost impossible to detect.
1932 - English physicist James Chadwick bombards beryllium with a-particles to knock out free neutrons, and thus becomes the first physicist to detect neutrons directly.
1932 - American physicist Carl Anderson is studying cosmic rays when he notices some tracks on his photographic plates that look exactly like electron tracks except that they are curving in the wrong direction. He realizes that that he has discovered a positively-charged electron, i.e., the antielectron predicted by Dirac. Anderson calls the new particle a positron.
Tech Note - Electrons and positrons are exactly alike, except that they have opposite charges and opposite quantum numbers. That, and one other little thing. If an electron and a positron touch, they instantly annihilate each other in a flash of g-rays. In other words, they are both converted into pure energy. This is why positrons don't last very long after they're created.
Star Trek Note - All particles have antiparticles, so there are also negatively charged antiprotons and so forth. Federation starships are supposedly powered by matter-antimatter reactions, which is probably why they are always blowing up so spectacularly. If Jordi lets his antimatter spill out of its magnetic container, he's in big trouble.
1934 - Frederic Joliot and his wife Irene Curie, daughter of Marie Curie, bombard aluminum with a-particles to produce phosphorus-30, the first artificially radioactive element.
1935 - Japanese physicist Hideki Yukawa proposes that the neutrons and protons in atomic nuclei are held together by an intensely powerful force which he calls the strong force. Working with the Dirac theory, he realizes that the fundamental forces must be carried by quanta, i.e., they cannot exist as classical "lines" of force. The only way for such quanta to exist and still be compatible with classical physics is if they "steal" their energy by popping in and out of existence so fast that conservation of energy is not violated because it is masked by the Heisenberg Uncertainty Principle. (In other words, the Uncertainty Principle applies even to empty space -- how do you know it's truly "empty", when the Principle won't let you measure its energy exactly?) Yukawa predicts that the strong force is "carried" by what he calls an "exchange particle". From the known sizes of atoms, and by assuming that the exchange particle usually moves near the speed of light, he calculates that it should have a mass about 200 times that of the electron.
1938 - It is now widely recognized that the calculation made by Hermann Helmholtz over 60 years ago, deriving an age of about 18 million years for the Sun, is far off the mark for exactly the same reason that Thompson's calculation for the age of the Earth was so far off: both the Earth and the Sun have nuclear energy sources. But the question remains: how does nuclear energy power the Sun? Its enormous energy yield is far too great to be created by traces of radioactive elements, as on Earth.
German-American physicist Hans Bethe calculates in detail how nuclear fusion, rather than nuclear fission, can power the Sun. He deduces a three-step sequence which we now call the proton-proton chain:
- Two protons collide so violently that a nuclear transformation takes place. One of the protons is converted into a neutron, and fuses with the other proton to form a deuteron, i.e., "heavy" hydrogen, 2H. To conserve charge and lepton number, an antielectron and a neutrino are emitted. The neutrino escapes from the Sun, but the antielectron immediately annhilates with an electron, releasing energy.
- The deuteron collides with a high-energy proton and the two fuse to form 3He. The mass of 3He is slightly less than that of 2H and a proton separately, and the excess mass is converted to high-energy gamma rays.
- Two energetic 3He atoms collide and in the resulting nano-nuclear fireball, an a-particle (4He atom) and two protons emerge. The mass difference before and after the collision is considerable: it releases approximately double the energy of the first two steps combined. The energy manifests itself primarily in the kinetic energy of the after-products, i.e., as heat.
- The net effect of the chain is that four hydrogen atoms have been converted into one helium atom, and 0.7% of the original mass of the hydrogen has been converted to energy. This corresponds to 175 million kilowatt-hours of energy from each kilogram of hydrogen.
1938 - Austrian physicists Otto Hahn and Lise Meitner bombard uranium with neutrons and discover nuclear fission. In short, uranium is a very large atom with over 230 protons and neutrons, so whacking it with a neutron "bullet" can cause it to split in two. Meitner, who is Jewish, flees to Sweden when Germany invades Austria and prepares a paper with the help of her nephew, physicist Otto Frisch. Frisch tells Bohr (see 1913) about the paper, who in turn spreads the word in the U.S. during a conference held in January, 1939.
1939 - Hungarian physicist Leo Szilard, having fled Nazi-occupied Europe for the U.S., learns of nuclear fission and realizes that it could be utilized to produce a chain reaction. He immediately begins a campaign to convince American scientists that they should voluntarily keep their nuclear research secret, so that the Nazis cannot learn from it. He is largely successful.
1940 - American physicists Edwin McMillan and Philip Abelson bombard uranium with neutrons to produce plutonium. Uranium is element number 92, and plutonium is element number 93, so McMillan and Abelson are the first physicists to produce a new element. In his efforts to isolate the plutonium, Abelson begins to develop methods to separate rare radioactive isotopes from their more common brethren. He has taken the first step towards producing enriched uranium.
 1941 - Acting partly in response to a letter signed by Albert Einstein and other prominent physicists, warning of the danger should Nazi Germany discover nuclear fission, President Franklin D. Roosevelt signs a secret order which starts the Manhattan Project.
1941 - Acting partly in response to a letter signed by Albert Einstein and other prominent physicists, warning of the danger should Nazi Germany discover nuclear fission, President Franklin D. Roosevelt signs a secret order which starts the Manhattan Project.
1942 - Enrico Fermi (see 1931), who by now has fled Fascist Italy for the U.S., is made the principal scientist responsible for producing a chain reaction for the Manhattan Project. Working in a secret laboratory located beneath the stands of the University of Chicago's football stadium, Fermi and his team construct the world's first nuclear pile (so called because it is literally a huge pile of carefully-arranged uranium, graphite, and cadmium blocks). At 3:45 pm on December 2, it is allowed to go critical for just a few seconds, proving that practical utilization of nuclear energy is possible. As a safety measure, three young physicists are standing on scaffolding above the pile with buckets of water containing dissolved cadmium salts -- they are told that they should pour their water into the pile if the reactor begins to have a run-away reaction. (In fairness, I must note that the pile also had a more conventional automatic shut-off device. But given that nobody had ever cranked up a reactor before, the team thought it best to play it safe.)
1945 - On July 16, just before dawn, the world's first atomic bomb is detonated at a test site in the desert 60 miles northwest of Alamogordo, New Mexico. Fermi makes an instant estimate of its power by tossing some paper bits into the air at the time of ignition, then observing how far the bits are blown by the blast. (Fermi was about 10 miles from ground zero.) This event follows three years of frenzied labor at secret facilities located in Hanford, Washington; Oak Ridge, Tennessee; and Los Alamos, New Mexico.
Barely a month later, atomic bombs nearly obliterate Hiroshima and Nagasaki, killing over 100,000 people. The Empire of Japan surrenders shortly afterwards. (The photo is of Nagasaki, Japan, on August 9, 1945.)
 Quantum Mechanics
|
Quantum & Nuclear Summary
Quantum Mechanics
|
Quantum & Nuclear Summary 
![]() Ideas of Physics Homepage
Ideas of Physics Homepage
A Ukrainian translation of this page is available here: Ukrainian